What is Vivistim Therapy™?
Vivistim Therapy can help break through chronic stroke limitations through the principles of neuroplasticity.
Vivistim® is the first FDA-approved neurostimulation device used in chronic ischemic stroke patients with moderate to severe arm impairment. It pairs vagus nerve stimulation (VNS) with upper extremity rehabilitation therapy and daily activities to help strengthen the brain connections needed to relearn motor tasks and increase neuroplasticity in the chronic stage.
- Paired VNS™ prompts the release of specific neuromodulators
- Improves the brain’s ability to relearn motor tasks
- Works within the brain to help the body during OT/PT

Boosting Therapy Results
Clinically Proven
2–3 times more hand and arm function compared to intense therapy alone1
FDA Approved Breakthrough Device
Approved by the FDA as safe and effective treatment for upper limb recovery after an ischemic stroke3
High Satisfaction
98% of Vivistim users were satisfied with their therapy2
Lasting Results
Improvements in function and reduction in impairment1,4
How Vivistim Works
Vivistim Therapy induces more neuroplasticity
-
Vagus nerve stimulation (VNS) activates the brain’s attention system by prompting the release of neuromodulators—acetylcholine, norepinephrine, serotonin.3
-
These neuromodulators interact with the motor neurons being activated during rehab
-
Works with high repetitions of salient, goal-driven tasks to induce motor learning.
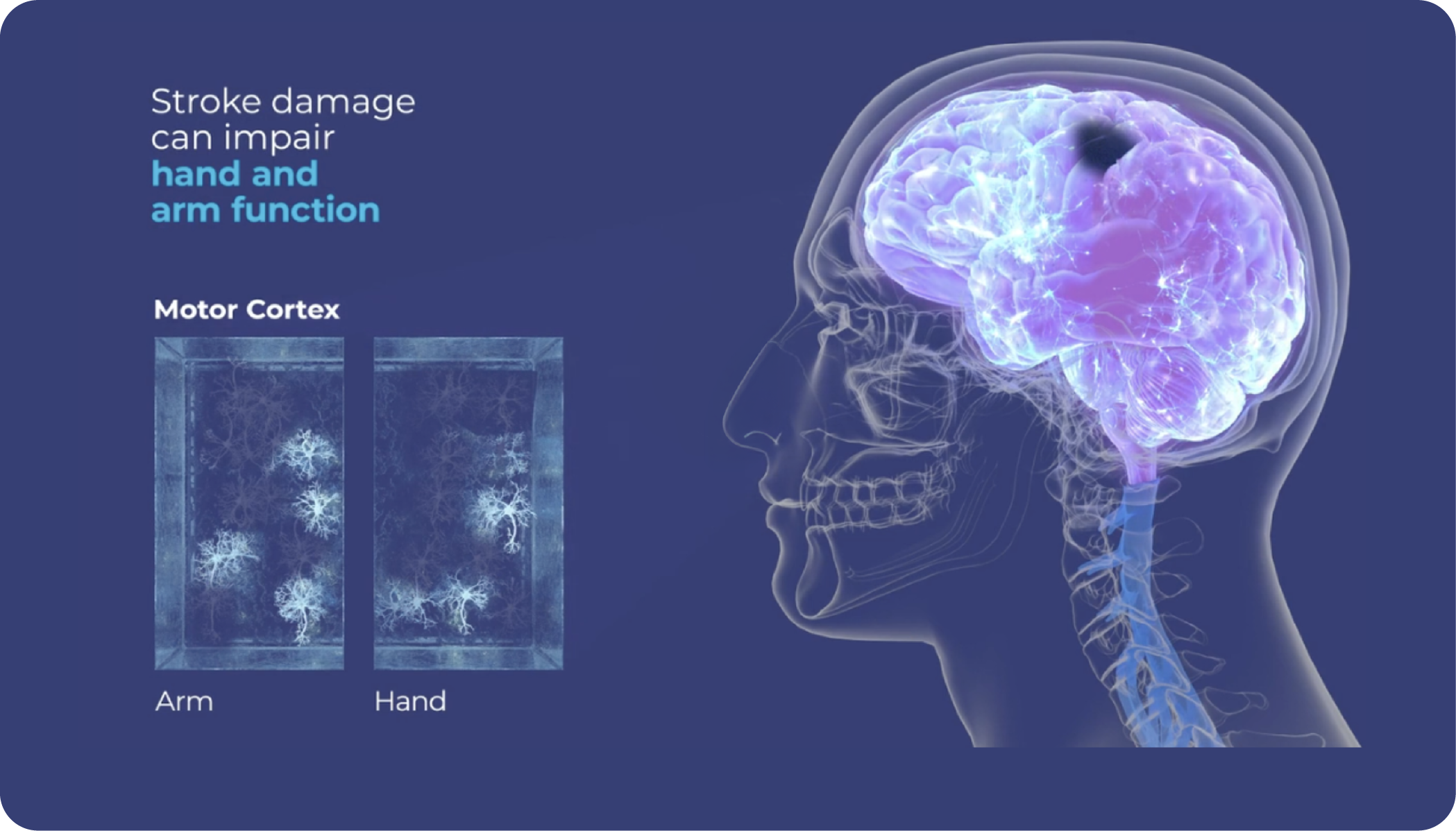
Vivistim Therapy is Proven Effective Across Multiple Studies
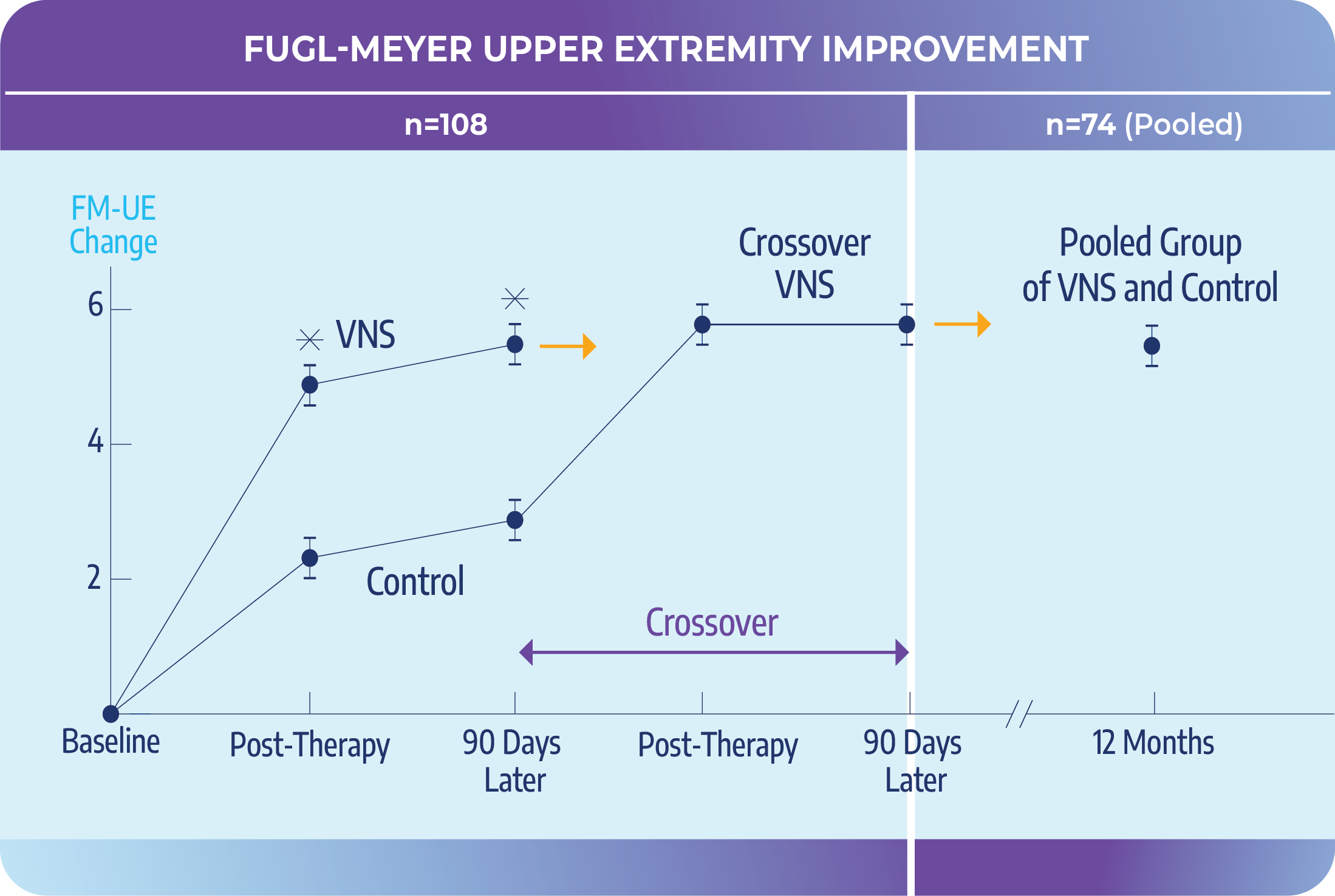
VNS-REHAB Pivotal Study, triple blind RCT
Active VNS vs Control
- Significant improvements in function and reduction of impairment compared with intense rehabilitation alone.
- The effects of Paired VNS were consistent across subgroups, including age, severity, and time post stroke.
Crossover VNS
- Crossover group matched VNS group outcomes once VNS was enabled.
Pooled Group by 1 Year
- Individuals treated with Paired VNS maintained long-term improvements in impairment, activity, participation, and quality of life at one-year.
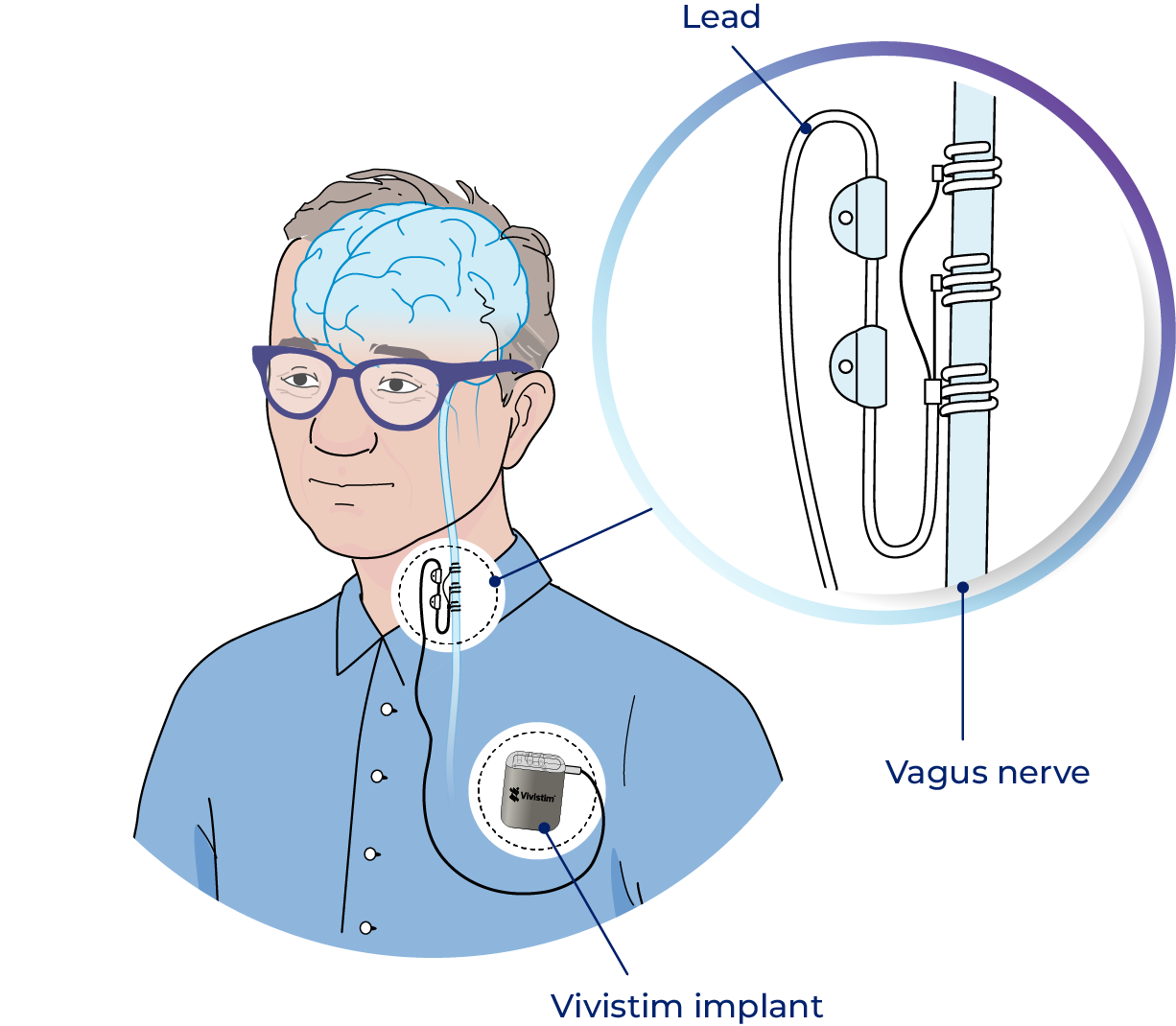
Vivistim Implant
Simple, outpatient procedure
The device is placed during a short outpatient procedure, typically by a neurosurgeon or other qualified implanting surgeon.
The generator is placed under the skin of the chest and the lead is tunneled from the neck to the generator.
Vivistim Therapy in Practice
More usage, more progress
In Clinic

- Therapist activates VNS during functional task-specific therapy
- Tasks should be salient, driven by each patient’s functional goals
- Sessions are 90 minutes, 3× per week for a minimum of 6 weeks
Out of Clinic
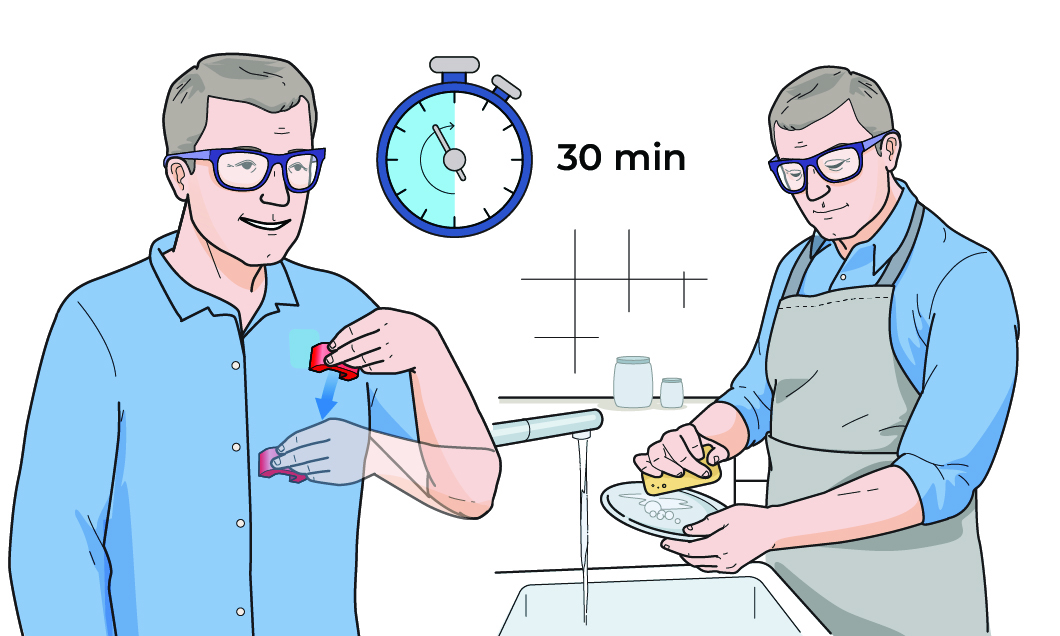
- Patients also activate VNS with a magnet while doing daily activities with their affected arm
- Self-activated sessions can be done up to 8× per day